|
Post-Embedding Antibody Staining for TEM
by Hall, Hartwieg and Nguyen
doi:10.3908/wormatlas.9.12
Summary Protocol
- Block thin sections held on nickel grids using 0.01M glycine in 0.1M phosphate buffer (PB); 6 x 1 min at rt.
- Block sections again with 0.5% gelatin, 0.1% nonfat dry milk,1% NGS (normal goat serum) in 0.01M glycine in 0.1M PB, 15 min at 37°C.
- Wash grids in 0.01M Glycine in 0.1M PB, 15 min, warmed to 37°C.
- Expose sections to primary antibody solution in 0.5% gelatin, 2 h, rt in the dark.
- Wash grids in 0.1M PB, 6 x 5 min, rt, on a slow shaker to achieve good washing action (see EMPostembedFIG 1).
- Expose sections to gold-linked secondary antibody diluted in 0.5% gelatin, 60 min, rt in the dark.
- Wash grids in 0.1M PB, 6 x 5 min as in step 5 above.
- Fix sections in 6.25 % glutaraldehyde in 0.1M PB, 5 min, rt.
- Wash grids in dH2O, 2 x 2 min.
- Air dry the grids and store in grid box.
- Prior to microscopy, stain grids in 4% uranyl acetate in H2O, 15 min, rt.
- Wash grids in dH2O, then allow to air dry.
Description
The development of techniques for applying gold-linked markers and antibodies at the TEM level has occupied workers for many years. Important developments follow early work of Bessereau (Rostaing et al., 2004) for postembedding techniques. We have reviewed postembedding techniques before (Hall, 1995). Advances in tissue preservation made possible by HPF/FS have also made better immunoEM results possible (Cueva et al., 2007; Ripper et al., 2008).
The following method can be used to test for the localization of an epitope at high resolution on plastic thin sections (Hall, 1995; Paupard et al., 2001). We recommend using either Lowicryl HM20 or LR Gold as the embedding resin, since these produce more wettable thin sections with better exposure of the epitope to a droplet of antibody solution compared to the Epon resins. We have had success using either microwave fixation or HPF + FS to prepare whole worms for this procedure. Microwave fixation has the advantage of giving much higher throughput of tissue, making it easier to mass many animals into a plastic block for simultaneous sectioning. However, the HPF method produces a better tissue ultrastructure (Cueva et al., 2007), especially for cell membranes or for active events such as endocytosis (Summary protocol).
Helpful Hints
Washes (steps #5 and 7) are done in 1 mL solution in a 24 well culture dish (covered to protect from dust) and placed on a tabletop shaker to agitate slowly (EMPostembedFIG 1).
 EMPostembedFIG 1 EMPostembedFIG 1
Spin the diluted secondary antibody for 4 min in a microfuge to remove any clumped gold conjugates before use in Step 6 above.
Grid transfer by platinum (or Nichrome) wire loop from solution to solution. If grids sink in the plastic well during washes, use a forceps with bent tips to grab them and move to next well.
Antibody reactions can be done on 5 µL drops on Parafilm in a humidified chamber. Incubations with antibody can be conducted at room temp, keeping the chamber closed and in the dark. Alternately, longer antibody incubations can be run at 4°C; this step again must be in a closed, humidified chamber to reduce any chance of drying effects.
A simple way to make a humidified chamber is to place a piece of wet paper towel beneath a fresh piece of Parafilm inside a plastic culture dish. Small antibody aliquots are positioned on the Parafilm, with one grid inverted onto each drop for antibody treatment, and the culture dish is kept closed during the incubation period. This can then be covered in foil, or placed in drawer to exclude light until the incubation is over.
Nickel grids should be coated with either Pioloform or Formvar (see Grid making). Mesh grids are commonly used, but coated nickel slot grids are also useful. Honeycomb mesh grids (thin bar style) offer the best combination of support and open area, and they are more stable than slot grids in resisting the rigors of an antibody treatment.
Troubleshooting
Be very certain that you know which side of the grid carries the
thin sections. You must always place that side of the grid down onto each drop of
solution for each step in the procedure.
Use a positive control antibody when first testing the procedure to affirm that you have all steps working properly (cf. Hall, 1995). We often use MH27 antibody to label adherens junctions (EMPostembedFIG 2), since those junctions can be found in almost any cross-section through the nematode body, whether in pharynx, hypodermis, or intestine.
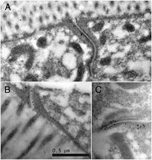 EMPostembedFIG 2 EMPostembedFIG 2
Compare several different dilutions of the primary antibody (see Hall, 1995; Paupard et al., 2001) to optimize specific binding versus nonspecific background binding. The primary antibody used in these experiments should be immunopurified prior to use to reduce nonspecific binding.
Cleanliness is essential in every step. The work area should be clean and quiet. Forceps should be cleaned just before use, fresh Parafilm should be used to hold drops of antibody during incubations, and all plasticware and glassware must be very clean.
Exact times in the protocol above are not very important, but the experiment should be well planned in advance to include a range of antibody dilutions that have been pre-tested by immunofluorescence. The blocking steps (#1 and #2 above) are essential to reduce non-specific binding.
Figures
 Click pictures for new window with figure and legend, click again for high resolution image Click pictures for new window with figure and legend, click again for high resolution image
EMPostembedFIG 1: Shaker table. Shaker table is used to hold 24-well culture dishes filled with rinse buffer for cleaning grids after immunoEM antibody processing steps. One treated grid is added for each row of 6 wells, and then serially transferred from well to well with a platinum wire loop. Samples are gently agitated on the shaker during each rinse step (per well).
EMPostembedFIG 2: ImmunoEM after microwave fixation. A, B, C. MH27Ab labeling adherens junctions in adult intestine. Gold-linked secondary Ab (black dots) indicate where MH27 Ab has bound to the thin section. From work of April Reedy, Pam Hoppe, and Ken Nguyen.
References
Cueva, J.G., Mulholland, A. and Goodman, M.B. 2007. Nanoscale organization of the MEC-4 DEG/ ENaC sensory mechanotransduction channel in C. elegans touch receptor neurons. J. Neurosci. 27: 14089–14098. Article
Hall, D.H. 1995. Electron microscopy and three-dimensional image reconstruction. Methods Cell Biol. 48: 395-436. Abstract
Hall, D.H., Hartweig, E. and Nguyen, K.C.Q. 2012. Modern electron microscopy methods for C. elegans. Methods Cell Biol. 107: 93-149. Abstract
Paupard, M.-C., Miller, A., Grant, B., Hirsh, D. and Hall, D.H. 2001. Immuno-EM localization of GFP-
tagged yolk proteins in C. elegans using microwave fixation. J. Histochem. Cytochem. 49: 949–95. Article
Ripper, D., Schwarz, H. and Srierhof, Y.-D. 2008. Cryo-section immunolabelling of difficult to preserve specmens: advantages of cryofixation, freeze-substitution and rehydration. Biol. Cell 100: 109–123. Article
Rostaing, P., Weimer, R.L., Jorgensen, E.M., Triller, A. and Bessereau, J.L. 2004. Preservation of immunoreactivity and fine structure of adult C. elegans tissues using high-pressure freezing. J. Histochem. Cytochem. 52: 1–12. Article
|
