|
Fine Structure of the Caenorhabditis elegans
Secretory-Excretory System
F. Kenneth Nelson, Patrice S. Albert, and Donald Riddle
Division of Biological Sciences, Tucker Hall
University of Missouri, Columbia Missouri, 65211 U.S.A.
Received August 24, 1982
J Ultrastruc. Res 1983 82:156-171; PMID: 6827646
DOI: 10.1016/S0022-5320(83)90050-3
Abstract -
Introduction -
Material & Methods -
Results -
Discussion -
Acknowledgments -
References
Abstract
The secretory-excretory system of C. elegans, reconstructed from serial-section electron micrographs of larvae, is composed of four cells, the nuclei of which are located on the ventral side of the pharynx and adjacent intestine. (1) The pore cell encloses the terminal one-third of the excretory duct which leads to an excretory pore at the ventral midline. (2) The duct cell surrounds the excretory duct with a lamellar membrane from the origin of the duct at the excretory sinus to the pore cell boundary. (3) A large H-shaped excretory cell extends bilateral canals anteriorly and posteriorly nearly the entire length of the worm. The excretory sinus within the cell body joins the lumena of the canals with the origin of the duct. (4) A binucleate, A-shaped gland cell extends bilateral processes anteriorly from cell bodies located just behind the pharynx. These processes are fused at the anterior lip of the cell, where the cell enters the circumpharyngeal nerve ring. The processes are also joined at the anterior edge of the excretory cell body, where the excretory cell and gland are joined to the duct cell at the origin of the duct. Secretory granules may be concentrated in the gland near this secretory-excretory junction. Although the gland cells of all growing developmental stages stain positively with paraldehyde-fuchsin, the gland of the dauer larva stage (a developmentally arrested third-stage larva) does not stain, nor do glands of starved worms of other stages. Dauer larvae uniquely lack secretory granules, and the gland cytoplasm is displaced by a labyrinth of large, transparent spaces. Exit from the dauer stage results in the return of active secretory morphology in fourth-stage larvae.
Introduction
The development and behavior of the free-living soil nematode, Caenorhabditis elegans, has been the subject of intensive study in recent years because of the advantages this simple metazoan offers for genetic, ultrastructural, and light microscopic analysis (reviewed by Riddle, 1978; Herman and Horvitz, 1980). This nematode develops by essentially invariant embryonic and post-embryonic cell lineages to produce an adult worm with fewer than 1000 somatic cells. The number and relative position of each of these cells with respect to one another is quite reproducible from animal to animal. Electron microscopy has proven to be a potent method for analysis of neural structure and development (White et al, 1978; Chalfie and Thomson, 1979; White et al, 1976); embryogenesis (Krieg et al, 1978), and the differentiation of specific tissues (Cox et al, 1981; Ward and Carrel, 1979).
Much of the fine structure of this nematode has been documented, including the anatomy of the neuro-muscular pharynx (Albertson and Thomson, 1976), the buccal capsule (Wright and Thomson, 1981), the anterior sensory anatomy (Ward et al, 1975; Ware et al, 1975), and the body wall musculature (Epstein et al,1974; Waterston et al, 1980).
Our study of the genetic and environmental controls on postembryonic development in C. elegans (Albert et al, 1981; Swanson and Riddle, 1981) led us to investigate the anatomy of the secretory apparatus of this nematode in order to lay the groundwork for additional studies on the possible role of secretory-excretory activity in molting (Singh and Sulston, 1978) or in dauer larva formation (Riddle et al, 1981). The dauer (German: dauer = enduring) larva is a developmentally arrested stage which may be formed at the second larval molt in response to starvation or overcrowding. Dauer larvae survive exposure to detergents and other chemicals (Cassada and Russell, 1975), do not feed, and may survive for months until they encounter food, molt, and resume development. Neural integration of specific chemosensory cues, one of which is a Caenorhabditis-specific pheromone, apparently determines developmental fate (Golden and Riddle, 1982), but nothing is known about the endocrine mechanisms that may be employed to trigger morphogenesis of the dauer larva.
Caenorhabditis elegans contains only a few cells exhibiting glandular ultrastructure. Five such cells in the pharynx open into the lumen via short ducts (Albertson and Thomson, 1976). These cells may be innervated coordinately with pharyngeal muscles, suggesting that they may secrete digestive enzymes in concert with pharyngeal pumping. It has also been suggested that these glands may be involved in molting (Singh and Sulston, 1978). A gland outside the pharynx is associated with the excretory system. Although the general features of excretory gland structure have been determined by light microscopy (Sulston and Horvitz, 1977; Mounier, 1981), structure-function relationships for this gland and the associated excretory system have not been studied. The absence of documented ultrastructure for these cells represents a gap in our understanding of the biology of this animal.
Several studies of secretory anatomy have been done in parasitic nematode species (Rogers, 1968; Waddell, 1968; Lee, 1970; Narang, 1972; Romanowski et al,1971; Davey and Hominick, 1973), but little is known about the physiology of these endocrine systems or the specific role they may play in development, reproduction, or behavior. Comparison of excretory systems among nematode species reveals that morphology is extremely variable. Some parasitic species apparently lack an excretory system altogether, at least during part of their life cycle (Chitwood and Chitwood, 1950). This variability suggests that the system has different functions in different species, and may be multifunctional in some. Osmoregulation is at least one function of the excretory cells in some species, because the rate of excretion is correlated with the osmotic strength of the medium (Weinstein, 1952; Croll et al., 1972). Although other possible functions such as waste-product clearance have not been rigorously established in any species, Ascarids have been shown to concentrate and expel injected dyes via the excretory duct (Behrenz, 1956). Because gland cells are associated with the excretory system in many species, it has been suggested that secreted products may be released to the outside environment through the excretory duct, or they may reach other internal tissues via the excretory canals (Romanowski et al., 1971). The excretory gland of the seal parasite, Phocanema decipiens, has been implicated in the release of peptidases involved in molting (Davey and Kan, 1968). Peptidases and esterases also have been identified in exsheathment fluid from other nematode species (Rogers, 1965). This paper describes the four cells found to be the structural components of the C. elegans secretory-excretory system. Cellular morphology has been reconstructed from electron micrographs of serially sectioned specimens. The nuclei of these cells are located in the head region on the ventral side of the pharynx, and on the ventral side of the intestine just posterior to the pharyngeal-intestinal valve. We have correlated changes in the physiology and developmental state of the nematode with changes in glandular morphology. In addition to the excretory gland, and duct cells previously described by light microscopic methods (Sulston and Horvitz, 1977; Mounier, 1981), we have characterized a specialized hypodermal cell, the "pore cell," which joins the excretory duct to the body-wall cuticle at the excretory pore. Also, we have characterized numerous subcellular morphological features which suggest specific physiological functions for each of the four cells. We have described a unique "secretory membrane" that joins the gland cell to the excretory system at the origin of the excretory duct. This membrane may selectively pass secreted products into the excretory duct or into the excretory sinus.
Materials & Methods
Growth of Nematodes
Wild-type C. elegans strain N2 was grown routinely at 15°C on NGM agar plates seeded with E. coli strain OP50 (Brenner, 1974). Synchronous second-stage larvae (L2s) were obtained by one of two methods. Eggs purified by alkaline hypochlorite treatment (Emmons et al, 1979) were rinsed, suspended in 1.0 ml M9 buffer (Brenner, 1974), and placed on a shaker at 25°C for 12-15 hr. The hatched first-stage larvae (L1 s) then were collected by centrifugation and put on petri plates with E. coli at 25°C. Alternatively, synchronous L2s were obtained from eggs laid by gravid adults during a 2-hr period. Larvae were fixed 2.5-3.0 hr after the L1-L2 molt, which was monitored by observation of pharyngeal pumping (Cassada and Russell, 1975). Starved L2s were prepared by suspending M9 buffer-rinsed larvae (collected 1 hr after the L1-L2 molt) in 1 ml buffer at 25°C for 3 days.
Starvation-induced dauer larvae were removed from NGM agar plates for fixation after 5 days of starvation. Nonstarved, pheromone-induced dauer larvae developed from eggs hatched at 25°C on agar medium made from autoclaved, clarified S-medium obtained from a previous nematode culture (Sulston and Brenner, 1974; Golden and Riddle, 1982). Any nondauers were removed after 2 days. Pheromone-induced dauer larvae were fixed 2-3 days after the L2-dauer molt. To obtain fourth-stage larvae (L4s), starvation-induced dauer larvae were put on a plate spread with E. coli at 25°C and allowed to recover. The L4s were fixed 3.0-3.5 hr after the dauer-L4 molt.
Larval stages or adults picked from an NGM plate were fixed with 1.0% OsO4 in 0.1 M sodium cacodylate-HCl, pH 7.3, for 1.5 hr at 28-30°C. Buffer-rinsed worms were cut in half and embedded in agar (Ward et al, 1975). Small agar blocks, each containing two specimens, were dehydrated in ethanol and embedded in Spurr's resin (Spurr, 1969). Transverse serial sections approximately 60 nm thick were picked up on unsupported slot grids, stained with uranyl acetate and lead citrate (Reynolds, 1963), and placed on lightly carbon-coated formvar films (Albert et al., 1981). Sections were photographed on a Philips 300 electron microscope.
Because reconstruction of the anatomy of C. elegans is simplified when the fixation method accentuates cell boundaries, fixation in osmium was preferred to the standard glutaraldehyde fix, osmium postfix method.
Glutaraldehyde-fixed specimens, however, were also examined to eliminate possible ambiguities in subcellular morphology. Larvae were fixed in 3% glutaraldehyde in .07 M sodium cacodylate-HCl, pH 7.3, at 4°C for 6 hr except for the first hour, which was at 20°C. Buffer-rinsed specimens were postfixed in cold 1% buffered OsO4 for 2.5 hr, during which time the temperature of the fixative was allowed to warm to room temperature (20°C).
Reconstruction
The reconstruction was prepared as a projection to a plane through the center of the organism. The ventral view was prepared by measuring the left-to-right distances of cell boundaries in the ventral half of the nematode from the sagittal plane, and projecting them onto the horizontal plane. The lateral view was prepared in the same way except dorsal-ventral distances were projected onto the sagittal plane. The measurements were taken on every twelfth section (every 720 nm) from the serial cross-section electron micrographs of a single L2 larva. The measurements were reproduced as points on paper, and continuous lines were drawn through the points representing the boundaries of cells or nuclei.
Nomarski Micrographs
Well-fed worms were washed off an NGM plate and transferred in 5 milimeterM9 buffer to a 5% agar pad supported on a glass microscope slide (Sulston and Horvitz, 1977). Before the worms were transferred to the slide, 5 millimeter of 100 mM NaN3, an anesthetic, were allowed to diffuse into the agar. The nematodes were observed through the differential interference-contrast optics of a Zeiss Universal microscope and photographed with an Olympus PM-10A camera. Kodak Technical Pan or Tri-X film was used, and all photographs were taken with a heat filter and a green filter in the light path.
Paraldehyde-Fuchsin (PAF) Staining
A mixed population of worms was washed from an NGM plate with M9 buffer and freed of bacteria by low speed centrifugation in distilled water. The animals were fixed for 3-7 days in modified Bouin fixative which was prepared with trichloroacetic acid. Staining was as described by Cameron and Steele (1959) except the oxidation was for 3 min in hot potassium permanganate.
The correlation of PAF staining with nutritional state of the animals was tested using L2 larvae synchronized as above by the alkaline hypochlorite method. One hour after the L1-L2 molt the worms were suspended in M9 buffer and incubated at 20°C for 20 hr without food. Half of the animals then were placed in fixative, and the other half were returned to food for 3.5 hr, washed free of bacteria, and fixed. Both the starved and the fed animals were PAF-stained. As a positive control in the staining procedure, a morphologically distinguishable C. elegans strain, CB61 (dpy-5 I), was mixed with each test sample at the time of fixation. In the mixed population with starved worms, about 90% of the positive control animals exhibited glandular staining, whereas none of the starved worms stained.
Results
Reconstruction
The organization of the C. elegans secretory-excretory system conforms generally to that found in other rhabditidae (Chitwood and Chitwood, 1950) and consists of four cells: (1) The large, H-shaped excretory cell extends bilateral canals anteriorly and posteriorly nearly the entire length of the worm. The cell body contains an excretory sinus, a system of small channels which joins the lumena of the four excretory canals with the origin of the excretory duct. (2) The duct cell surrounds the cuticle-lined excretory duct from the origin of the duct to the pore cell boundary. A lamellar system, formed by multiple invaginations of the duct cell plasma membrane, greatly increases the surface area of the cell at the duct lining. (3) The pore cell encloses the terminal one-third of the duct and underlies the excretory pore at the ventral surface of the animal. (4) A binucleate, A-shaped gland cell extends bilaterally symmetrical processes from cell bodies just behind the terminal bulb of the pharynx anteriorly to the nerve ring where the processes join and apparently receive synaptic input. The gland cell processes are also joined across the anterior edge of the excretory cell body, where the gland cell, duct cell, and excretory cell are all joined at a tight junction. We call this point of intercellular transfer the secretory-excretory junction.
The reconstruction of secretory-excretory anatomy from transverse serial-section micrographs of a second-stage (L2) larva is shown in Figs. 1-4. These results were confirmed in a second specimen. The L2 stage precedes the molt at which dauer larvae may be formed and provides an appropriate point of reference for anatomical comparisons with dauer larvae. In Figs. 1-4, both lateral and ventral projections have been divided into two drawings for clarity. Figures 1 and 2 show ventral and lateral views of the gland and excretory cells together with an outline of the excretory duct. Figures 3 and 4 show the duct and pore cells. The figures show a portion of the worm beginning at the circumpharyngeal nerve ring approximately 65 micrometer behind the tip of the nose and ending just behind the pharyngeal-intestinal valve. This represents a length of about 30 micrometer or 8% of the total body length of a second-stage larva. We have also examined these cells in dauer larvae, L4 larvae recovered from the dauer stage, and in adults. Some aspects of subcellular organization become more elaborate with increasing maturity, although the secretory-excretory system is presumably fully functional at the time of hatching, or very soon thereafter.
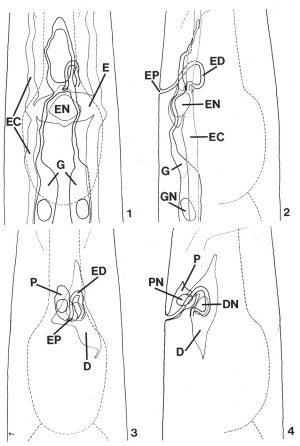 FIGS. 1-4. Reconstruction of C. elegans secretory-excretory anatomy from transverse serial-section electron micrographs of a second-stage (L2) larva. The area shown encompasses 30 micrometer beginning approximately 65 micrometer from the tip of the nose. Lateral and ventral projections have been divided into two drawings each for clarity. Outlines of the pharynx and posterior portion of the nerve ring (thin dashed lines) have been included for reference. X 1900.
FIGS. 1-4. Reconstruction of C. elegans secretory-excretory anatomy from transverse serial-section electron micrographs of a second-stage (L2) larva. The area shown encompasses 30 micrometer beginning approximately 65 micrometer from the tip of the nose. Lateral and ventral projections have been divided into two drawings each for clarity. Outlines of the pharynx and posterior portion of the nerve ring (thin dashed lines) have been included for reference. X 1900.
FIG. 1. Ventral view of the gland cell (G), the excretory cell (E), and the excretory duct (labeled in Fig. 2). Excretory canals (EC), excretory cell nucleus (EN).
FIG. 2. Lateral view of the cells shown in Fig. 1. Excretory pore (EP), excretory duct (ED), excretory cell nucleus (EN), excretory canal (EC), gland cell (G), gland cell nucleus (GN).
FIG. 3. Ventral view of the duct cell (D) and pore cell (P). Excretory duct (ED), excretory pore (EP).
FIG. 4. Lateral view of the region shown in Fig. 3. Pore cell (P), pore cell nucleus (PN), duct cell (D), duct cell nucleus (DN).
Many features of secretory-excretory morphology are visible in Nomarski interference-contrast microscopy. Figures 5 and 6 show two different focal planes of the region around the terminal bulb of the pharynx in an L4 larva. These light micrographs show the excretory cell nucleus, pore cell nucleus, excretory duct and excretory pore along the ventral midline (Fig. 5), and the duct cell nucleus and one of the gland cell nuclei just above the focal plane of the excretory cell (Fig. 6). The granular cytoplasm of the gland cell and the highly refractile secretory-excretory junction are also visible. A large nucleolus is visible in each nucleus.
Excretory Cell
The excretory cell is an exceptionally large cell, the nucleus of which is located on the ventral side of the terminal pharyngeal bulb (Fig. 5). The cell body forms a bridge which connects the subventral, bilateral excretory canals (Fig. 1). This is in contrast to some nematodes, such as Ascaris, in which the bilateral canals are connected only by a small duct (Chitwood and Chitwood, 1950). Much of the cell body is occupied by the large nucleus (Fig. 7).
The excretory canals are fluid-filled channels surrounded by the cytoplasm of the excretory cell processes. These processes, which extend both anteriorly and posteriorly from the excretory cell body, form gap junctions with the adjacent hypodermis (Fig. 9). The plasma membrane surrounding the canal does not form junctions with other tissues such as adjacent neurons or muscle cells, but the canal membranes are exposed to the pseudocoelom. Within the canals are canaliculi (small dead-end channels) which usually appear as small circles in the transverse-section micrographs (Fig. 9). The canaliculi, which are contiguous with the lumen of the canals, increase surface area. Mitochondria are a prominent feature of the canal cytoplasm. At the cell body, the canals fuse to form a sinus just anterior to the excretory cell nucleus (Fig. 8). This excretory sinus joins with the excretory duct at its origin (Figs. 8, 15), where the excretory cell, gland cell, and duct cell form a complex, branched tight junction (Figs. 16, 17) morphologically similar to the zonula occludens in vertebrate epithelial tissue. In addition to forming gap junctions with the hypodermis via the lateral canals, the excretory cell also forms gap junctions with the duct cell and the pore cell (Fig. 16).
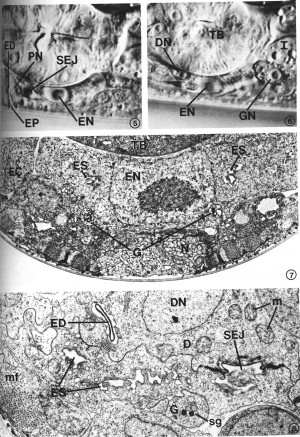 FIGS. 5-6. Nomarski micrographs taken at two focal planes through the secretory-excretory anatomy of a fourth-stage (L4) larva. X 1940. FIG. 5. Excretory duct (ED), excretory pore (EP), pore cell nucleus (PN), excretory cell nucleus (EN), secretory-excretory junction (SEJ). FIG. 6. Focal plane slightly above that of the excretory cell nucleus (EN). Duct cell nucleus (DN), one of the gland cell nuclei (GN), terminal pharyngeal bulb (TB), intestine (I).
FIGS. 5-6. Nomarski micrographs taken at two focal planes through the secretory-excretory anatomy of a fourth-stage (L4) larva. X 1940. FIG. 5. Excretory duct (ED), excretory pore (EP), pore cell nucleus (PN), excretory cell nucleus (EN), secretory-excretory junction (SEJ). FIG. 6. Focal plane slightly above that of the excretory cell nucleus (EN). Duct cell nucleus (DN), one of the gland cell nuclei (GN), terminal pharyngeal bulb (TB), intestine (I).
FIG. 7. Excretory cell. The cell body is characterized by a prominent nucleus (EN) and sinuses (ES) which join the excretory canal (EC) lumena in more anterior sections. Gland cell process (G), neurons (N), terminal pharyngeal bulb (TB). L2. X 13 300.
FIG. 8. Detail of excretory cell sinus (ES) and duct cell (D). The sinus is open to the excretory duct at the secretory-excretory junction (SEJ). Although this L2 had been starved for 3 days prior to fixation (see text), excretory cell morphology was not appreciably affected, whereas body wall muscle filaments (mf) show considerable disorganization. Duct cell nucleus (DN), excretory duct (ED), gland cell process (G), mitochondria (m), secretory granules (sg). X 21 300.
Duct Cell
The duct cell is adjacent to the pharynx at the antero-ventral side of the terminal bulb. Its nucleus, containing a prominent nucleolus (Fig. 10), is located just anterior and lateral (either left or right) to the excretory cell body (Fig. 6). The cell cytoplasm is rich in mitochondria (Fig. 8). The duct cell completely surrounds the excretory duct from its origin to the boundary of the pore cell. The excretory duct is a channel lined with a collagenous cuticular wall and the plasma membrane of the duct cell (Fig. 10). The duct wall is continuous with the body-wall cuticle at the excretory pore. The duct forms a loop through the cell so that, in the L2, a distance of only 2 micrometer is traversed by a 9-micrometer segment of the duct (Fig. 2). The looped path taken by the duct is such that a single transverse section may transect the duct as many as four times.
The cytoplasm around the excretory duct is bordered by stacks of parallel lamellar membranes (Fig. 10). The sheetlike membrane stacks are invaginations of the plasma membrane surrounding the duct. Small vesicles are occasionally associated with these membranes. The looped path of the duct through the cell, combined with extensive lamellar folding of the adjacent cell membrane dramatically increases the surface area of the cell's interface with the duct. The lamellar profiles become more elaborate in specimens of greater maturity, so that they eventually become a dominant feature of the cell in an older adult (not shown). The duct cell of an L4 larva (Fig. 10) exhibits an intermediate degree of lamellar proliferation.
Observations of live nematodes with Nomarski interference-contrast microscopy reveal that the excretory duct of the dauer larva pulsates. Periodic swelling of the duct, beginning at its origin, is followed by a release of fluid through the excretory pore. We have not detected any musclelike structures within the duct cell, even at high magnification of glutaraldehyde-fixed specimens, so duct pulsation may be nonmuscular.
Pore Cell
A pore cell has been described in other nematode species (Narang, 1972; Dick and Wright, 1974), but C. elegans provides an example of an excretory duct contained by two cells, a duct cell and a pore cell. The structural involvement of the pore cell in C. elegans was beyond the resolution of previous histological studies (Mounier, 1981). The point at which the excretory duct is transferred from the duct cell to the pore cell is shown in Figs. 11-13. The duct cell forms a tight junction with the pore cell at the point where the duct is transferred. The pore cell surrounds the duct by wrapping around it and forming a tight junction with itself (Fig. 13). This intracellular tight junction follows along the duct to the excretory pore. In this manner the pore cell encloses the terminal one-third of the duct (a distance of about 5 micrometer in an L2 larva) and joins the duct with the external cuticle at the excretory pore (Fig. 16).
The pore cell divides the nerve bundle which connects the circumpharyngeal nerve ring with the retrovesicular ganglion. It underlies the body-wall cuticle in the immediate region around the excretory pore (Figs. 16, 17). In the dauer larva, a stage resistant to treatment with detergents and other chemical agents (Cassada and Russell, 1975), the excretory pore remains open as it is in other stages. The cuticular lining of the dauer pore lacks the radially striated layer (Fig. 20), which is characteristic of the dauer body-wall cuticle (Popham and Webster, 1978; Cox et al, 1981).
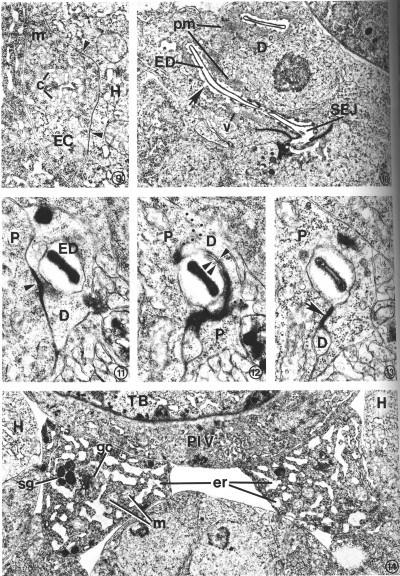 FIG. 9. Transverse section through the left posterior excretory canal (EC) in an L2. Canaliculi (c), small dead-end channels, surround and are contiguous with the canal lumen, center. Gap junctions (arrow heads) form between the excretory canal and adjacent hypodermis (H). Mitochondrion (m). X 20 500.
FIG. 9. Transverse section through the left posterior excretory canal (EC) in an L2. Canaliculi (c), small dead-end channels, surround and are contiguous with the canal lumen, center. Gap junctions (arrow heads) form between the excretory canal and adjacent hypodermis (H). Mitochondrion (m). X 20 500.
FIG. 10. Duct cell (D) in an L4 which developed from a dauer larva. Cytoplasm surrounding the cuticle-lined excretory duct (ED) is characterized by stacks of parallel membranes (pm) which are continuous with the plasma membrane surrounding the duct (arrow). Small vesicles (v) are occasionally associated with the membrane stacks. Secretory-excretory junction (SEJ). X 12 500.
FIGS. 11-13. Excretory duct transition between the duct cell and pore cell of an L2. X 34 400.
FIG. 11. Excretory duct (ED) entirely within the duct cell (D). Pore cell (P). Duct cell-pore cell junction (arrowhead).
FIG. 12. Three sections posterior to that shown in Fig. 11. The excretory duct is just inside the pore cell (P). The duct cell membrane (single arrowhead) and pore cell membrane (double arrowhead) are apposed, but no longer within the tight junction. Obliquely sectioned membranes have been marked with dots to emphasize boundaries between portions of the duct (D) and pore cells.
FIG. 13. Two sections posterior to that shown in Fig. 12. The pore cell (P) has surrounded the excretory duct and formed a tight junction (arrow) with itself. The small duct cell process (D) terminates within the next four sections.
FIG. 14. Excretory gland cell body morphology. The cytoplasm is characterized by an extensive network of rough endoplasmic reticulum (er) which takes on a sinuslike appearance due to the dilated cisternae. Small clusters of electron-dense secretory granules (sg) are observed, frequently in close proximity to Golgi complexes (gc). Ribosomes and mitochondria (m) are abundant. The area shown is located just anterior to the gland nuclei. Hypodermis (H), terminal pharyngeal bulb (TB), pharyngeal-intestinal valve (PIV). L2. X 1 5 300.
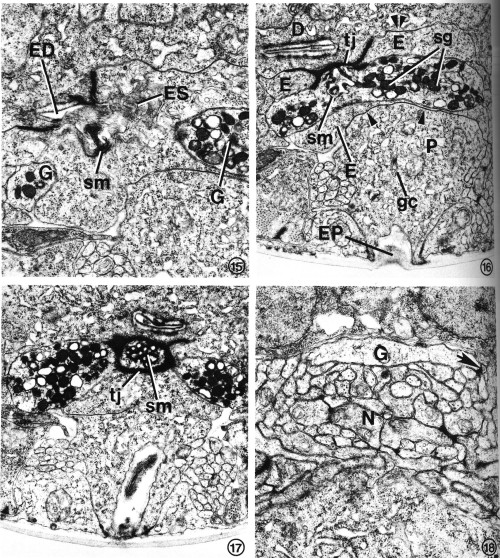 FIGS. 15-18. Excretory gland morphology at selected regions anterior to the cell bodies. Transverse-section micrographs of L2s proceed from posterior to anterior.
FIGS. 15-18. Excretory gland morphology at selected regions anterior to the cell bodies. Transverse-section micrographs of L2s proceed from posterior to anterior.
FIG. 15. At the secretory-excretory junction, part of the gland cell secretory membrane (sm) is exposed to the origin of the excretory duct (ED). Filaments protruding from the excretory cell sinus (ES) can be seen in the duct. The two gland cell processes (G) are also visible. X 23 100.
FIG. 16. The gland processes fuse, forming a cytoplasmic bridge rich in secretory granules (sg). This portion of the excretory gland is bound to the duct cell (D) and excretory cell (E) by a complex tight junction (tj). Gap junctions join the excretory cell to the duct cell (double arrowheads) and pore cell (single arrowhead). Pore cell (P), excretory pore (EP), Golgi complex (gc), secretory membrane (sm). X 17 800.
FIG. 17. The cytoplasmic bridge region reveals that the secretory membrane (sm) can be oriented such that the channels are cut transversely rather than longitudinally. In this orientation, the tight junction (tj) encircles the secretory membrane. X 17 000.
FIG. 18. A single excretory gland process (G) is formed by the fusion of the two anterior gland processes (see Fig. 1) just before entry into the base of the circumpharyngeal nerve ring. The gland cell presumably receives synaptic input from one or more neurons (N). One possible junction appears as a dense plaque (arrow). X 22 100.
The pore cell cytoplasm completely lacks the lamellar sheets found in the duct cell. The cytoplasm, however, contains numerous mitochondria, rough endoplasmic reticulum, and Golgi complexes (Fig. 16), suggesting the cell may secrete the cuticle around the pore. The pore cell forms an extensive gap junction with the excretory cell (Figs. 16, 17).
Excretory Gland Cell
The excretory gland cell is a binucleate, A-shaped cell whose bilateral, subventral nuclei are located just posterior to the pharyngeal-intestinal valve (Figs. 1, 2, 6). An extensive network of endoplasmic reticulum (ER) is the most prominent feature of the cell body cytoplasm (Fig. 14). The dilated cisternae of the rough ER give the cell a sinuslike appearance. The cytoplasm is densely populated with ribosomes and contains numerous mitochondria. Small clusters of electron-dense granules are frequently found in close proximity to Golgi complexes. These membrane-bound granules are presumably transported anteriorly and are concentrated where the bilateral processes fuse, forming a granule-filled bridge across the anterior edge of the excretory cell body (Figs. 15-17). Several classes of secretory granule can be distinguished on the basis of electron density in osmium-fixed specimens. A uniquely specialized portion of the gland cell membrane, the "secretory membrane" (Figs. 15-17), is connected to the origin of the excretory duct. We have not observed granules within the duct or in the excretory sinus.
Anterior to the granule-filled bridge of the gland cell, the bilateral processes separate again but are fused a second time just prior to entry into the base of the circumpharyngeal nerve ring (Fig. 18). Presumably, the gland cell receives synaptic input from one or more of the many neurons surrounding the anterior tip of the cell. One possible synaptic junction can be seen as a dense plaque in Fig. 18.
The gland cell can be stained with the histochemical reagent paraldehyde-fuchsin (PAF). The portion of the cell which stains most intensely is the region where secretory granules are most concentrated (Fig. 23). Although PAF-staining is sometimes considered to be specific for neurosecretory cells, we cannot resolve any PAF-positive neurons in C. elegans. The only tissues found to stain positively are the excretory gland cell, the lining of the pharynx, the body-wall cuticle and, rarely, the pharyngeal gland (g1 and g2) cell bodies (Figs. 23, 24).
Secretory-Excretory Junction
The secretory-excretory junction, centered at the anterior edge of the excretory cell body, is the point at which the gland and the excretory cell converge with the duct cell at the origin of the excretory duct. A tight junction binds the three cells together (Figs. 16, 17). The junction, surrounded by a diffuse band of dense cytoplasm, is pentilaminar, and 90-110 Angstrom wide in four animals from which measurements were taken (high-magnification micrograph not shown). The two adjacent membranes appear to be fused or very closely apposed.
At the extreme posterior origin of the duct, the excretory sinus opens directly into the open channel. This area contains an accumulation of filaments of varying lengths and orientations which protrude from the sinus into the duct (Figs. 8, 15). Directly anterior and adjacent to this point, the gland cell is connected to the excretory duct via the secretory membrane (Figs. 15, 20). Thus, any glandular secretions entering the duct conceivably may also enter the excretory sinus (Fig. 20). The gland-duct junction is between the postero-dorsal side of the gland cell "bridge" and the ventral edge of the excretory duct. The secretory membrane is encircled by a tight junction (Fig. 17). Its orientation with respect to the axis of the worm is quite variable, so that it was sectioned longitudinally in Fig. 16, but transversely in Fig. 17. A similar comparison between such orientations can be made between Figs. 19 and 20. Figures 16, 17, and 19-22 all show portions of the secretory membrane and the adjacent tight junction in slightly different orientations. In some orientations, the secretory membrane appears to be structured into an assembly of "secretory channels." The membrane-enclosed channels are about 40-50 nm in diameter (Fig. 19), and are lined with amorphous, dense material.
Physiological and Developmental Variations in Excretory Gland Morphology
In L2 and post-dauer L4 larvae, and in adults, numerous secretory granules are concentrated in the gland cell near the secretory-excretory junction. Similar granules are associated with Golgi complexes in the cell bodies. The gland cell enlarges in proportion to the size of the growing animal, and adults contain many more granules than do young larvae. In two L2s and in two post-dauer L4 larvae examined, the majority of the secretory granules are extremely osmiophilic, whereas in two adults examined, a greater proportion of the granules are of intermediate and low electron density (not shown).
Dauer larvae totally lack secretory granules. Whether the dauer stage is induced by starvation (Fig. 19) or by exogenously added pheromone in the presence of abundant food (Fig. 20), the gland cell cytoplasm appears to contain only a loose membranous network. In the gland cell bodies, the nuclei, mitochondria, and ribosomes are visible but are narrowly confined within a fibrous cytoplasmic network as if the cisternae of the endoplasmic reticulum swelled to occupy all available extraorganellar volume. Near the secretory-excretory junction, the intracellular network may be ER, or the membranous residue of depleted secretory vesicles, or a combination of both. Because the absence of secretory granules was observed in two starvation-induced and two pheromone-induced (nonstarved) dauer larvae, we conclude that the change in gland morphology is correlated with the developmental state itself, rather than the nutritional condition of the animal prior to dauer larva formation. We also examined the gland in an L2 larva that had been starved by incubation in M9 buffer for 3 days, the approximate age of the dauer larvae studied. Although a reduced number of secretory granules was observed in the starved L2 (Figs. 8, 21), the cellular morphology remained similar to that of well-fed worms (Figs. 15-17). Thus, starvation alone does not account for the type of vacuolation unique to the dauer larva gland.
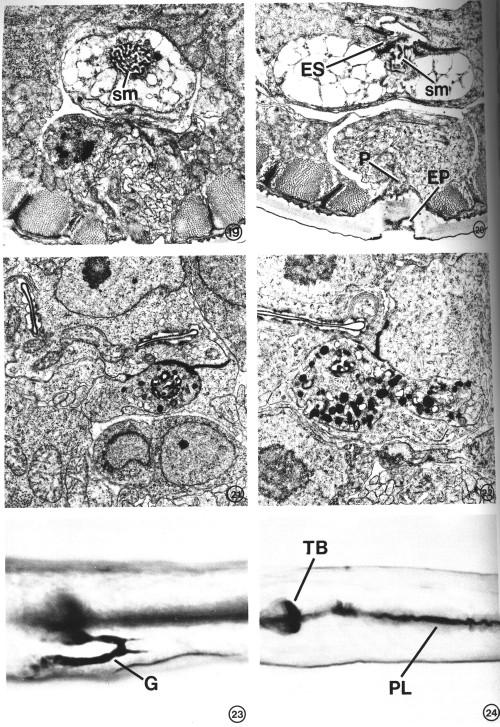 FIGS. 19-22. Variation in excretory gland morphology correlated with the physiological state and/or developmental stage. X 15 000.
FIGS. 19-22. Variation in excretory gland morphology correlated with the physiological state and/or developmental stage. X 15 000.
FIG. 19. The excretory gland of a starvation-induced dauer larva (see text) totally lacks the secretory granules normally observed in an L2 (see Figs. 15-17). This portion of the gland contains only a membranous network of cytoplasm in addition to the secretory membrane (sm). The morphology of the secretory membrane does not differ from that of the L2.
FIG. 20. Gland cell morphology of a dauer larva induced in the presence of food (bacteria) by exogenous application of a Caenorhabditis-specific pheromone (see text). Gland morphology is like that observed in the starvation-induced dauer larva (Fig. 19). This section also shows the excretory cell sinus (ES) and the gland secretory membrane (sm) simultaneously open to the duct and to each other. Excretory pore (EP), pore cell (P).
FIG. 21. Excretory gland of an L2 starved for 3 days prior to fixation. Secretory granules are present, though in reduced number, and the cytoplasmic morphology is similar to that observed in well-fed L2s (see Figs. 16, 17).
FIG. 22. Examination of the first post-dauer developmental stage (L4) reveals a return to predauer (L2) gland morphology.
FIGS. 23 AND 24. Light micrographs of paraldehyde-fuchsin (PAF) stained nematodes. Animals were grown asynchronously on plates seeded with bacteria and stained as described under Materials and Methods. X 1075.
FIG. 23. PAF-positive gland cell (G) of a nonstarved adult. The body-wall cuticle and pharyngeal lining also stain.
FIG. 24. An L4 starved for 36 hr lacks PAF staining in gland cell. Terminal pharyngeal bulb (TB), pharyngeal lining (PL).
The post-dauer fate of the gland cell also was examined (Fig. 22). Starvation-induced dauer larvae were placed in food and allowed to molt and develop into L4s. Examination of two specimens revealed that a subcellular morphology rich in secretory granules similar to those in the L2 stage was regained.
In contrast to dauer-specific elimination of secretory granules, staining of the gland cell with PAF seems to be highly dependent on nutritional state as well as developmental state. The gland cells of all growing developmental stages are strongly PAF-positive (Fig. 23). Body-wall cuticle and the pharyngeal lining also are stained. Although pharyngeal and cuticular staining are exhibited by dauer larvae, the excretory gland does not stain (not shown). Furthermore excretory gland staining is eliminated in any larval stage that has been starved for as little as 6 hr prior to fixation. A PAF-stained L4 larva, starved for 36 hr, is shown in Fig. 24. Again, pharyngeal and cuticular staining are not affected under these conditions. The loss of PAF staining is readily reversible. In one experiment, staining was restored to more than 90% (109 of 117 animals observed) of the L2 larvae that were fed E. coli for 3.5 hr after 24 hr of starvation. The ability of the gland to be stained with PAF may be correlated with a high density of secretory granules or with a higher level of gland cell activity than may be typical of either dauer larvae or starved animals per se.
Discussion
We have described the ultrastructure of the C. elegans secretory-excretory system. This four-cell organ develops embryonically with the exception of the pore cell (designated G2p) which surrounds the excretory duct early in the second larval stage, replacing an embryonic pore cell, G1 (Sulston and Horvitz, 1977). The entire system is descended from the early embryonic stem cell, AB, which gives rise to primary ectoderm (von Ehrenstein and Schierenberg, 1980; Sulston, White, Thomson, Schierenberg, and von Ehrenstein, personal communication).
Functions which have been attributed to nematode excretory systems are (1) excretion of metabolic waste, (2) osmoregulation, (3) secretion of molting exsheathment fluid, and (4) secretion and export of hormones to target tissues. The excretory cell of C. elegans has a morphology consistent with any or all of these functions. The excretory canals are exposed to the pseudocoelom, and they form extensive gap junctions with the hypodermis. The energy required for active transport across canal membranes could be made available by the abundant mitochondria within the canal cytoplasm, and the surface area at which exchange may take place is increased by canalicular branching of the lumena. The exchange process conceivably could work in either direction, to excrete wastes into the canals or to resorb, perhaps selectively, substances from the lumena. Any substances collected in the excretory canals are presumably transported from the organism's body via the excretory sinus, through the excretory duct to the excretory pore. In addition, it is possible that substances may be excreted from the hypodermis directly through the cuticle, which is water permeable in all stages.
In other nematode species, osmoregulation is perhaps the best documented excretory function. Weinstein (1952), Croll et al., (1972), and Atkinson and Onwuliri (1981) observed that changes in the rate of excretory duct pulsation correspond with changes in the osmolarity of the environment. In general, the more sudden the change toward a hypotonic medium, the faster were the pulsations of the duct. Waddell (1968) observed excretory duct pulsation in L3 (infective stage) larvae of Stephanurus dentatus (pig kidney worm), but fluid from L4s or adults was excreted without duct pulsation. Excretory duct pulsation also has been observed in Panagrellus and Heterodera (Narang, 1972).
Under routine laboratory conditions, we were able to detect pulsation of the excretory duct only in dauer larvae. It is possible that only relatively fast rates of excretion involve duct pulsation. In C. elegans, a rapid change in water balance occurs at the L2-dauer molt, when radial shrinkage of the body results in an increased density of the dauer larva relative to the L2. Electron micrographs reveal that the volume of the hypodermis is preferentially reduced. The excretory system may be involved in the establishment or maintenance of this state. Thus, dauer larvae may employ excretory pulsing to maintain an appropriate water balance, whereas other stages of lower density may not need to do so. Additionally, the specialized dauer larva cuticle may be less permeable to excreted products, imposing a greater demand on the dauer excretory system.
We observed no structural features suggesting neuromuscular control of excretory duct pulsation in dauer larvae. Instead, the normally collapsed duct may be periodically forced open by internal pressure from the excretory cell. Once accumulating fluid has opened the duct along its entire length to the excretory pore, internal pressure would be quickly released, and the duct could collapse once again. The extensive folding of the duct cell plasma membrane around the duct may provide for the rapid expansion and contraction of the duct during excretory pulsing. Similar lamellar folds surrounding the excretory duct also have been observed in Panagrellus redivivus (Narang, 1972).
A second possible function of the duct cell membrane is exchange of materials between the duct contents and the duct cell cytoplasm. The duct cell may selectively reabsorb salts or other substances entering the duct. The lamellar membrane amplifies the cell surface area at the duct, and abundant mitochondria within the duct cell could provide the required energy for active transport. We would not propose such a function for the pore cell, because its plasma membrane lacks the lamellar structure.
The morphology of the gland cell obviously suggests that a large amount of material is synthesized within this cell, packaged into granules, secreted into the duct, and transported to the exterior of the worm along with fluid from the excretory sinus. The presence of secretory granules in all growing stages examined suggests that secretion is either constant or occurs at repeated intervals. At least some, if not all, of the secretory granules are membrane-bound. If they release their contents into the duct by fusing with the plasma membrane of the gland cell at the secretory-excretory junction, the apparent complexity of the secretory membrane at this point may simply reflect the accumulation of membrane in the restricted area which is entirely bound by a tight junction. We do not know whether the type of tight junction found in the C. elegans secretory-excretory system is functionally identical to the zonula occludens found in vertebrate epithelium. However, it meets the major morphological criteria established for tight junctions in vertebrates. Permeability of these structures to heavy metal tracers has not been tested.
The consistent morphology of the secretory membrane in animals of various developmental and physiological states suggests that this structure may be an organelle. Although some of the dense material associated with the secretory membrane may be deposited during fusion with the electron-dense granules, none of our micrographs actually show granules fusing with the secretory membrane, nor have we seen portions of the membrane budding from the cell. The secretory membrane retains its dense, convoluted, tubular morphology even in dauer larvae, which lack the secretory granules altogether. Examination of previously published light micrographs (Romanowski et al., 1971) indicates that a similar structure may exist in other nematodes as well.
Studies on Phocanema decipiens indicate that a molting hormone acts on the excretory gland to bring about release of a "molting fluid" through the excretory pore into the space between the old cuticle and the newly synthesized, underlying cuticle (Davey, 1966; Davey and Kan, 1968). A leucine aminopeptidase was identified as a component of the molting fluid, and it was proposed that such enzymes may be involved in release or partial degradation of the discarded outer cuticle. The morphology of the gland in C. elegans also is consistent with a role in secretion of molting enzymes. The results of laser ablation of the gland cell, duct cell, or excretory cell nucleus in L2 larvae, however, led Singh and Sulston (1978) to conclude that the C. elegans excretory system is not essential for molting. These workers observed the loosening of the older cuticle to begin at the tip of the head rather than at the excretory pore. Furthermore, the amount of secretory granules in the excretory gland does not cycle with the molts, but increases steadily during development (with the exception of the dauer stage), and the greatest number of granules are found in the adult. The gland cells of all feeding stages of C. elegans (including the adult) are PAF-positive, whereas the glands of starved animals and dauer larvae are not. The ultrastructural comparisons suggest that PAF staining is correlated with gland activity.
The gland may not be essential for development or reproduction in the laboratory. Nevertheless, it may function in many ways to enhance survival in the natural soil environment. One substance released by C. elegans during all growth stages is a pheromone that in crowded cultures enhances entry into, and inhibits exit from, the dauer larva stage (Golden and Riddle, 1982). This pheromone or other informational molecules could be secreted via the excretory duct.
The gland cell ultrastructure does not preclude the possibility that substances may be secreted into the pseudocoelom, either by direct diffusion across the plasma membrane, or by fusion of secretory granules with the plasma membrane at sites other than the secretory-excretory junction. In addition, secreted products could be transported throughout the body of the animal via the excretory canals.
The striking differences in gland cell morphology between dauer larvae and other developmental stages suggest that release or degradation of secretory granules accompanies dauer formation, and that secretory activity is not present in the dauer stage- It also seems likely that gland function is not required to initiate exit from the dauer stage. The elimination of secretory granules and vacuolation of the cell is correlated with the dauer state itself and not with nutritional condition. No cause and effect relationship between dauer formation and gland function, however, has yet been established. We plan to perform laser microsurgery experiments, in which both gland cell nuclei will be destroyed during the first larval stage, in order to test the possible effects of cell ablation on dauer larva formation.
Acknowledgments
This work was supported by DHHS Grant HD11239 and by Research Career Development Award HD00367 to D.L.R. from the National Institute of Child Health and Human Development. F.K.N. is a predoctoral trainee supported by DHHS Grant GM07494. We thank Dr. Victor Dropkin, Dr. William Krause, and Dr. Robert Waterston for helpful discussions.
References
ALBERT, P. S., BROWN, S. J., AND RIDDLE, D. L. (1981) J. Comp. Neurol. 198, 435-451.
ALBERTSON, D. G., AND THOMSON, J. N. (1976) Philos.Trans. R. Soc. London Ser. B 275, 299-325.
ATKINSON, H. J., AND ONWULIRI, C. O. E. (1981) Exp.Parasitol. 52, 191-198.
BEHRENZ, W. (1956) Z. Wiss. Zoo/. Abt. A 159, 129-164.
BRENNER, S. (1974) Genetics 77, 71-94.
CAMERON, M. L., AND STEELE, J. E. (1959) Stain Tech-nol. 34, 265-266.
CASSADA, R. C., AND RUSSELL, R. L. (1975) Dev. Biol.46, 326-342.
CHALFIE, M., AND THOMSON, J. N. (1979) J. Cell Biol.82 278-289.
CHITWOOD, M. B., AND CHITWOOD, B. G. (1950) Introduction to Nematology, pp. 126-135, Univ. ParkPress, Baltimore.
COX, G. N., STAPRANS, S., AND EDGAR, R. S. (1981)Dev. Biol. 86, 456-470.
CROLL, N. A., SLATER, L., AND SMITH, J. M. (1972) Exp. Parasitol. 31, 356-360.
DAVEY, K. G. (1966) Amer. Zool. 6, 243-249.
DAVEY, K. G., AND HOMINICK, W. M. (1973) Exp.Parasitol. 33,212-225.
DAVEY, K. G., AND KAN, S. P. (1968) Canad. J. Zool.46, 893-898.
DICK, T. A., AND WRIGHT, K. A. (1974) Canad. J. Zool.52, 245-250.
EMMONS, S. W., KLASS, M. R., AND HIRSH, D. (1979) Proc. Nat. Acad. Sci. USA 76, 1333-1337.
EPSTEIN, H. F., WATERSTON, R. H., AND BRENNER, S. (1974) J. Mol. Biol. 90, 291-300.
GOLDEN,J W., AND RIDDLE, D. L. (1982) Science 218,578-580.
HERMAN, R. K., AND HORVITZ, H. R. (1980) in ZUCKERMAN, B. M. (Ed.), Nematodes as Biological Models, Vol. 1, pp. 227-261, Academic Press, New York.
KRIEG, C., COLE, T., DEPPE, U., SCHIERENBERG, E.,SCHMITT, D., YODER, B., AND VON EHRENSTEIN, G. (1978) Dev. Biol. 65, 193-215.
LEE, D. L. (1970) Tissue Cell 2, 225-231.
MOUNIER, N. (1981) Nematologica 21, 160-166.
NARANG, H. K. (1972) Parasitology 64, 253-268.
POPHAM, J. D., AND WEBSTER, J. M. (1978) Canad. J. Zool. 56, 1556-1563.
RIDDLE, D. L. (1978) J. Nematol. 10, 1-16.
RIDDLE, D. L., SWANSON, M. M., AND ALBERT, P. S.(1981) Nature (London) 290, 668-671.
REYNOLDS, E. S. (1963) J. Cell Biol. 17, 208-212.
ROGERS, W. P. (1965) Comp. Biochem. Physiol. 14, 311-321.
ROGERS, W. P. (1968) Parasitology 58, 657-662.
ROMANOWSKJ, R. D., THOMPSON, D. E., AND MADDEN,P. A. (1971) Proc. Helminthol. Soc. Wash. 38, 143-149.
SINGH, R. N., AND SULSTON, J. E. (1978) Nematologica 24, 63-71.
SPURR, A. R. (1969) J. Ultrastruct. Res. 26, 31-43.
SULSTON, J. E., AND BRENNER, S. (1974) Genetics 77,95-104.
SULSTON, J. E., AND HORVITZ, H. R. (1977) Dev. Biol. 56, 110-156.
SWANSON, M. M., AND RIDDLE, D. L. (1981) Dev. Biol. 84, 27-40.
VON EHRENSTEIN, G., AND SCHIERENBERG, E. (1980) in ZUCKERMAN, B. M. (Ed.), Nematodes as BiologicalModels, Vol. 1, pp. 1-71, Academic Press, New York.
WADDELL, A. H. (1968) Parasitology 58, 907-919.
WARD, S., AND CARREL, J. S. (1979) Dev. Biol. 73, 304-321.
WARD, S., THOMSON, J. N., WHITE, J. G., AND BRENNER, S. (1975) J. Comp. Neural. 160, 313-338.
WARE, R. W., CLARK, D., CROSSLAND, K., AND RUSSELL, R. L. (1975) J. Comp. Neurol. 162, 71-110.
WATERSTON, R. H., THOMSON, J. N., AND BRENNER, S. (1980) Dev. Biol. 77, 271-302.
WEINSTEIN, P. P. (1952) Exp. Parasitol. 1, 363-376.
WHITE, J. G., ALBERTSON, D. G., AND ANNESS, M. A.R. (1978) Nature (London) 271, 764-766.
WHITE, J. G., SOUTHGATE, E., THOMSON, J. N., AND BRENNER, S. (1976) Philos. Trans. R. Soc. LondonSer. B 275, 327-348.
WRIGHT, K. A., AND THOMSON, J. N. (1981) Canad.J. Zoo. 59, 1952-1961.
|